Earn Your M.S in Pharmacy – International Biomedical Regulatory Sciences
Join the highly skilled Regulatory Sciences professionals who work to ensure safer medical products and therapies around the world.
100% Online

Asynchronous Coursework
Non-Thesis
Esteemed Faculty
Contact us

John Mayhall (M.S '21)
The faculty and staff of UGA’s online programs are welcoming, friendly, available, and willing to do what is necessary to help you succeed. I believe in the human touch and have felt a lifelong obligation to help others in any way possible. I hope to use my abilities to navigate members of the industry through regulatory compliance matters so that most people can receive the most therapeutic relief and the best medical treatment and diagnosis as possible.
About the Program
Offered through the University of Georgia’s College of Pharmacy, the online M.S. in International Biomedical Regulatory Sciences provides the strong professional background needed to succeed in administrative positions and specialized areas that are required of the growing number of multi-disciplinary, hands-on professions in regulatory affairs.
UGA's regulatory sciences master is uniquely positioned to cover requirements in both the medical and veterinary industries. This program is a non-thesis/project-based master's and is ideal for working professionals who have a clear objective to cultivate a career in regulatory affairs, and for those with industry backgrounds who desire an advanced education in regulatory management.
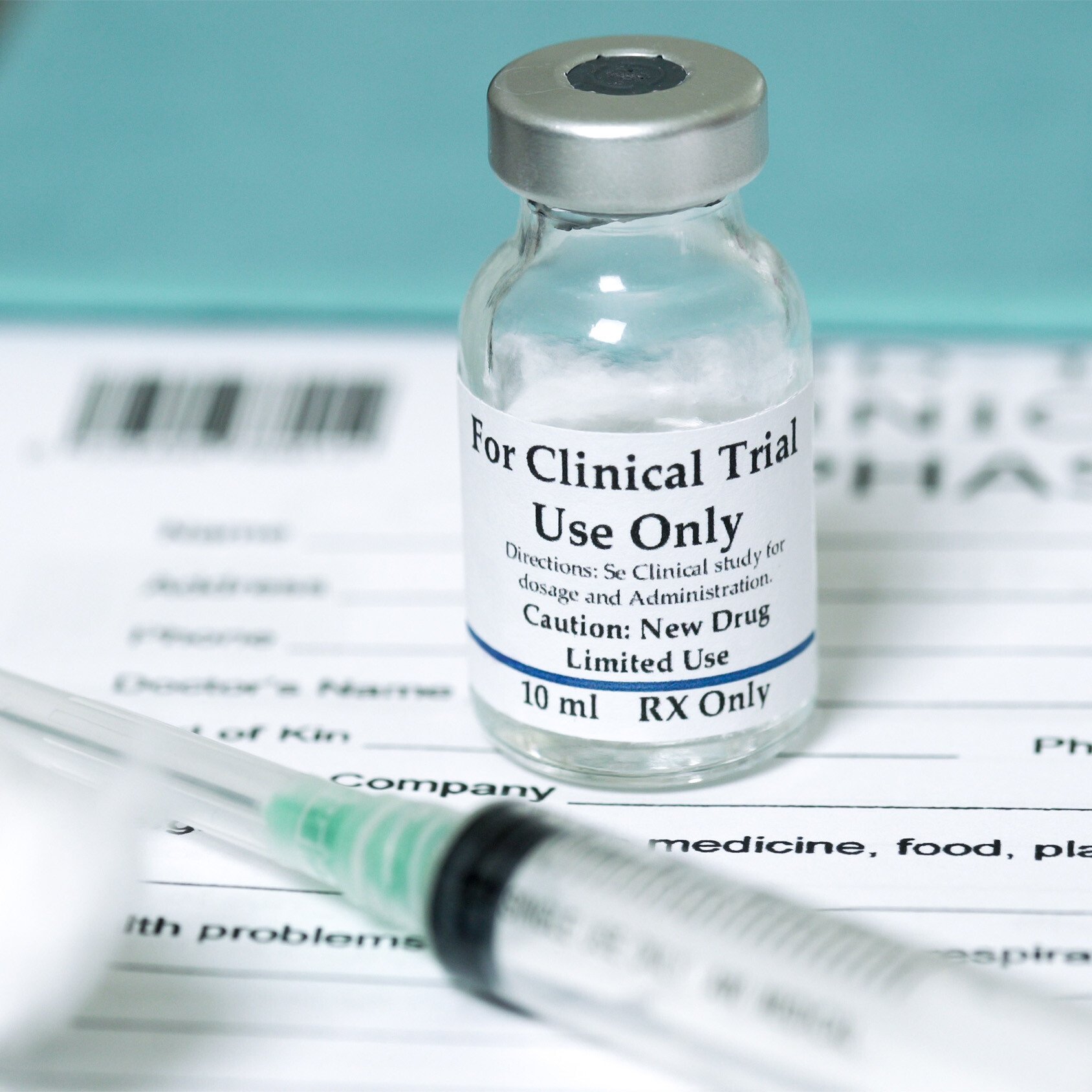
The courses cover regulatory requirements for Pharmaceutical, Biologic, Medical devices, Animal Health, International Regulations, and Combination Products as well as the management and monitoring of clinical trials.
*A thesis option is available but it requires additional time to complete.
Find out what you can achieve with an online degree from UGA by requesting more information now!
Become a Bulldog, On Your Own Time
Whether online or on-campus, earn the same prestigious UGA degree, while prioritizing the learning style and timing that's right for you.
Asynchronous courses can be completed as your busy schedule permits, whether that means during lunch break at your 9-5, on the weekends before watching the big game, or at night when the family has gone to bed. The path to success in the Bulldog Nation is yours to define.

M.S in International Biomedical Regulatory Sciences

Choose a Practical Non-Thesis Pathway
The non‐thesis pathway will engage you in the real‐world application, practice, development, and experiential learning needed for career success. These practical aspects of the job are highly desired by employers and the non‐thesis pathway is particularly suitable for students who do not plan to pursue research-intensive positions or continue on to doctoral studies.

Designed for industry professionals
This program is ideal for individuals who have a clear objective to pursue a career in regulatory affairs, as well as those with an industry background desiring an advanced education to thrive in regulatory management.
Explore a variety of career options
Regulatory science professionals are employed in industry, government, and academia and provide a range of services related to the regulation, development, manufacturing, and marketing of pharmaceuticals, medical devices, in-vitro diagnostics, biologics, biotechnology, nutritional products, cosmetics, and veterinary products.
Contact us
Interested? Learn more!
Contact our Enrollment Coach to learn more about the program, ask questions, and apply today.


